- Point of view
From master data management to business data services
Discover a better way to turn data into insights

The ability to turn data into insights is critically important to helping enterprises prepare for the future. As consumer behavior evolves, demand fluctuates, employee needs change, and businesses must make rapid, data-driven decisions to adapt and thrive. However, many organizations struggle to unleash the potential of their data.
Some look to master data management (MDM) to create a single source of consistent, accurate data for generating insights. Though MDM programs address many master data governance requirements, a wide variety of data – like reference data, relationship data, and market data – is left out. Without a comprehensive view of all types of data, business problems cannot be fully analyzed, and this limits the ability to deliver insights at speed. This is where business data services (BDS) comes in.
BDS, the next generation of MDM, integrates processes, technology, and talent to create an operating model in which data is available on demand for a variety of use cases. With BDS, all employees can access a rich catalog of data services to solve critical business problems.
Seizing the opportunity
BDS represents a major shift in how organizations provision and consume data to uncover actionable insights. With a robust BDS strategy, you can:
- Identify missed revenue opportunities
- Reduce total cost of data ownership
- Improve data literacy to smooth change management
- Increase agility in responding to market changes
- Enhance your ability to deliver data as a service
Putting theory into practice
BDS has helped a leading consumer packaged goods company increase the speed at which it launches new products. Initially, the company was trying to solve the problem by examining one area of product launch data. It soon realized it needed data from the entire supply chain to make informed decisions about how to build a new, faster product launch operating model. BDS connected this data through intelligent data capture, dynamic process orchestration, and KPI modeling to deliver the insights required to significantly reduce launch cycle time.
In another example, a Fortune 500 company wanted to use analytics to make more data-driven decisions at speed and scale. As part of its BDS strategy, the company established an internal data governance organization. This organization collated its data and developed learning programs to increase data literacy among employees. Now, every employee across multiple business functions can turn data into insights.
Taking ownership of data
Building an effective BDS strategy takes a concerted effort across multiple business functions and different business leaders. However, it's not always an easy ride. Here are five challenges that organizations often face without business alignment:
- Distributed data operating models across functional silos that lead to rework and reinventing data for each project
- Limited skill sets causing employees to manage and manipulate data without relevant business context
- Lack of governance, which creates multiple and conflicting versions of the truth
- Lack of a consolidated data process as business functions often focus solely on the data they own and ignore the complementary data needed to execute a transaction
- Limited return on investment when organizations are unable to connect the dots between data quality and business benefits
Many of these challenges occur when gaps exist between global business services (GBS) leaders and data and analytics leaders. Though both sides of the business are responsible for working with data, differences in their responsibilities (table 1) and focus areas can limit collaboration.
Table 1: The limitations of siloed data ownership
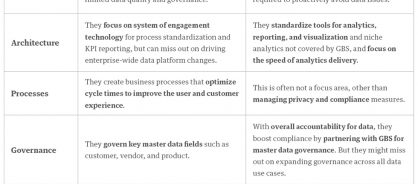
So, how do you close the gaps?
Aligning your organization
To align your enterprise across the five key components of BDS – operations, architecture, processes, governance, and culture – adopt a BDS framework (figure 1) that connects data, processes, technology, and talent. GBS leaders – or really any business function with access to data – should feel confident in collaborating with data and analytics leaders to unlock insights to make informed decisions that support a range of business benefits.
Figure 1: The business data services framework
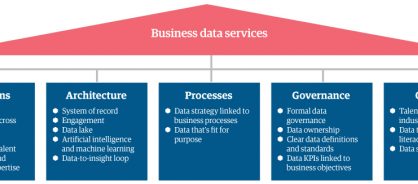
Within this framework, you'll align your master data – as well as analytics, artificial intelligence (AI), and machine learning (ML) capabilities – to rapidly transform data into actionable insights. Bilingual talent – employees who can speak the language of both business process and technology – are another piece of the puzzle. They'll look at data in relation to different business processes to ensure they are using it in a way that unlocks maximum benefits for your business.
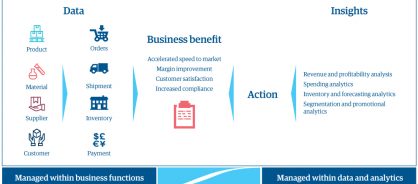
When you mature from MDM to BDS, you eliminate the silos between functions who have access to data and the functions who turn data into insights (figure 2). This leads to a better employee experience, more insights into what customers want and need, increased agility, and enhanced compliance.
Figure 2: Breaking down silos to turn data into insights
Beginning your journey
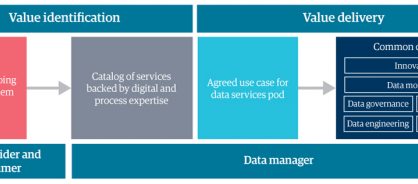
The journey from MDM to BDS begins with identifying a good use case – a one-off or ongoing business problem that data-driven insights can address (figure 3). When you find a use case, you can bring data services experts together as a team, or pod. These pods focus on innovation and developing data modeling, governance, quality, engineering, and AI and ML capabilities to solve the business problem.
Figure 3: The business data services operating model
Before embarking on your journey and launching a BDS operating model, consider these pointers:
- Prioritize data use cases that will unlock insights for multiple business functions – the more employees who will benefit, the higher-priority the use case
- Develop a data services playbook to make sure every employee understands how to access and manage data within a transparent and thoroughly governed process
- Execute a pilot use case to test out new data hypotheses before making huge investments in an idea
- Showcase your success to the wider organization to increase confidence in BDS
As the world around us changes constantly, the ability to act on data-driven insights will separate leaders from laggards. It's time for enterprises to move from MDM to BDS, bringing data, processes, technology, and talent together to act and adapt with agility in the face of whatever challenge the future holds.