- Solution overview
Hitting the ground running after an M&A with full compliance to all known regulations
Make your transition seamless with integration expertise and technology

The pharmaceutical industry now accounts for more than $200 billion in mergers and acquisitions (M&A) annually – the most of any sector in the current economy. Before realizing the many efficiencies M&As bring, life sciences companies must first deal with issues such as integration delays, supply disruptions, and noncompliance thanks to multiple connected teams operating in silos.
Challenge
Dealing with regulatory, supply, and quality challenges
Straight off the mark, these reconfigured businesses need to focus on key value creation in areas such as sales and marketing, distribution networks, and product development.
Yet several factors that slow down their efforts can affect their brand image badly when targets aren't met. Inefficient, imported legacy siloed operations, for example, can cause integration bottlenecks and disconnects between manufacturing initiatives, product release goals, and regulatory approval timelines. Sometimes, third-party distributor contracts and licenses even get lost in the shuffle.
So do the hoped-for benefits of the M&A. At the end of the day, Genpact estimates that choke points like these, which impact supply-chain efficiency, data quality, and processes for compliance, typically cost companies 1 percent of the acquisition product revenue following an M&A. That's a bad precedent to set.
Solution
A plan with advisory services, a sharp new tool, and an integration mindset
Our M&A consultancy has well-tested techniques to tackle these roadblocks to growth. We guide companies right from the earliest stages of their M&A, all the way through ongoing submissions, reporting, and compliance maintenance.
Here's how we help. We take a cross-functional approach that combines regulatory affairs, manufacturing, and data quality into a single entity. This smart, interconnected system helps you avoid repeat submissions, manage supply chain changes such as product releases aligned with regulatory timelines, and ensure that regulators are getting the detailed information they need for approvals. And the robust governance process we devise with you tracks and effectively manages the merger. You get a clear line of sight and full transparency from the first integration steps until the moment an updated product enters the market.
Our work encompasses three phases. Phase one entails fact-gathering to assess the impact of the M&A. Then, we go to work planning. We determine the status of licenses, clinical trial applications, and SKUs, outlining actions such as transfers or other changes that each will need. This helps us set priorities, such as getting products that are medically necessary to market quickly, while keeping revenue goals in mind. Next, we establish a budget estimate for managing integration. Finally, we work with people in regulatory, manufacturing, and quality functions to draw up global, regional, and local governance forums.
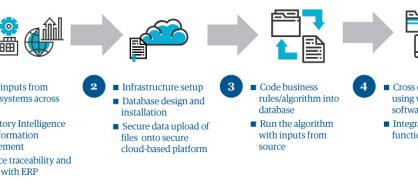
In the next phase, we act on what we've learned. While we develop and refine submission plans and provide help updating paperwork, we work with people in regulatory, supply, and quality to set timetables for applications, reports, and products. MAST, our marketing authorization supply transfer solution, which integrates planning and implementation across the regulatory and supply functions, makes that job easier. This secure, cloud-based analytical tool with built-in regulatory intelligence as an accelerator captures critical cross-functional milestones and links marketing applications with supply SKUs. It generates an integrated view on its dashboard and can even simulate future scenarios, identify gaps associated with the distribution of products, and track workloads in the finest detail. It provides an end-to-end integrated view across regulatory, safety, and quality and prevents revenue loss (see figure 1).
Meanwhile, we conduct forums on governance to ensure that your company is managing risks and issues proactively and keeping close track of all reports.
Phase three is the maintenance stage. We connect with all distributors and national representatives to keep compliance requirements and submissions up to date. At the same time, we monitor the migration of data to make sure it is clean, centralized, and harmonized in go-forward systems.
Figure 1: Genpact's proprietary MAST tool provides integrated planning & implementation capability across regulatory, supply and quality
Impact
Better resource efficiency and enhanced insights
The holistic approach we take to managing M&As for pharma pays off in a number of ways. The cross-functional system we institute helps you plan workloads and resource allocation far more efficiently, giving all stakeholders clear visibility based on real-time insights. That can help prevent supply gaps and enhance decision-making in general.
This service also provides the confidence that your company is performing its due diligence in regulatory and quality functions. By virtually guaranteeing 100% compliance with all known regulations, we eliminate the prospect of penalties.