- Solution overview
Transforming CMC Regulatory Affairs in life sciences, for better compliance and operating efficiency

As regulatory complexity continues to increase across global markets, and growth through mergers and acquisitions continues, Chemistry, Manufacturing and Controls (CMC) organizations are overwhelmed with the volume of activity. Re-thinking the operating model, and leveraging external expertise is an opportunity to solve capacity challenges and meet the demands of the business.
The growth and throughput of current CMC organizations cannot be sustained in today’s dynamic industry environment
- Increasingly stringent regulatory requirements: Across the globe, health authorities continue to raise the bar on the documentation required for review to achieve regulatory approvals. The level of detail that must be continuously tracked from the initial clinical trial, throughout the pre-registration, registration, post-approval lifecycle is putting greater demands on CMC regulatory professionals
- Merger and acquisition activity impact: This increases the number of submissions to reflect changes in the source of supply, plant closures and product site transfers
- Resource management is not optimized: As regulatory requirements are becoming more complex, so more in-depth knowledge is required, forcing a rise in staffing needs, and creating a talent crunch. The operating model is not generally optimized to staff for peaks during high-volume submissions, nor can organizations hope to retain all regional specific regulatory expertise in-house. Expensive, high-caliber staff often perform low-value, repetitive tasks, unable to focus on strategically important activity
Take a copy for yourself
Strategic outsourcing enables CMC organizations functions to execute globally with efficiency at scale
Many best-in-class organizations are rethinking their regulatory operating model to meet these challenges.Outsourcing is increasingly part of the formula for accessing needed CMC regulatory capabilities. This is because a strategic partnership with an outsourcing provider can deliver considerable business impact, such as improved compliance (ability to keep pace with evolving market-specific regulatory requirements), scalable resourcing for variability in demand, and greater efficiency and effectiveness through improvement in process efficiencies and cost reduction across the function.
Reimagine the regulatory affairs operating model with transformational CMC support
Genpact Regulatory Affairs is the industry’s leading global regulatory services provider and a specialist in end-to-end CMC services. Delivering global regulatory affairs support for nearly two decades, we deliver end-to-end global regulatory affairs services through all phases of the product lifecycle – from new product filing strategy to all aspects of lifecycle management.
Leveraging our global CMC teams and capabilities in process, technology, and analytics, our skilled professionals work closely with clients to provide holistic regulatory solutions supporting CMC strategy, CMC authoring, lifecycle submissions, and dossier review and compliance (See Figure 1).
Figure 1: CMC Regulatory Affairs - Solution portfolio
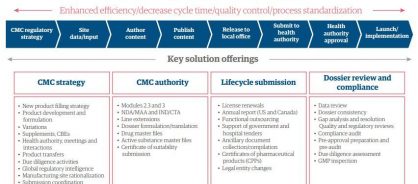
Genpact Regulatory Affairs enables clients to source scalable regulatory operations services, and transition to an operating model that meets their business needs:
Stage 1: IMPROVE the current regulatory operating model with a Genpact Regulatory Affairs regulatory solution that focuses on cost-effective regulatory services combined with process and technology tools to achieve incremental productivity improvements. With CMC services, this may focus on routine lifecycle management activities, such as variations, achieving efficiency improvements of 20-30%;
Stage 2: With a regulatory solution that consolidates operations across business divisions clients can TRANSFORM the operating model. CMC services in each business unit are transitioned to global hubs, which specialize and standardize operations, realizing 2-3x the efficiency benefits;
Stage 3: A strategic outsourcing partnership with Genpact Regulatory Affairs, where maintenance activities for a set of mature products are externalized, can SUSTAIN efficiency benefits of 3-10x.
Genpact Regulatory Affairs can lead clients at any stage through meaningful transformation, and tailor the service model to their unique business requirements. This includes technical staffing, project consulting, or sustainable, full-service outsourcing. Our industry experts can determine the solution that meets current operational needs, or support clients in evolving their operating model, leveraging the right mix of process, technology and analytics to achieve new levels of operational efficiency and performance.
Why Genpact Regulatory Affairs?
As specialists in regulatory affairs, Genpact Regulatory Affairs has nearly two decades of experience and a track record of providing high quality regulatory services, driving the entire regulatory application submission lifecycle, from new drug application to lifecycle maintenance. We are uniquely positioned to deliver integrated regulatory affairs solutions for life sciences companies:
- Deep domain expertise: Providing high quality, global CMC and regulatory affairs services to 8 out of 10 of the leading life sciences companies, our seasoned team of industry experts has extensive regulatory experience across all major and emerging markets
- Scalable, global delivery footprint: Leverage scalable, cost-effective resources from our team of regulatory professionals, strategically situated in centers of excellence around the world
- Flexible delivery models: Tailored services to meet any organization’s specific needs with on-and off- and near-shore resources for greater efficiency and maximum value
- Local regulatory insight: Leverage our proprietary Pharmalink Affiliate Network (PAN) to provide local regulatory affairs expertise in the local language in any regulated market. Covering 166 markets globally, the PAN provides local regulatory insight, strategy and intelligence in markets not covered by a global delivery center
- Standardized regulatory processes: Our proprietary Smart Enterprise ProcessesSM is used to define robust, standardized regulatory processes that leverage industry best practices, minimizing waste and rework for maximum efficiency
- Advanced regulatory technologies: Applying new technologies, such as Systems of EngagementTM for regulatory, enables a unified process workflow to maintain data integrity and provides real-time performance analytics
Case study
End-to-end CMC outsourcing support for a major pharmaceutical company
Challenge
Major pharmaceutical company was aiming to reduce costs by rationalizing their workforce, while balancing priorities between new product development and lifecycle management of mature products in a dynamic CMC regulatory environment.
Solution
Genpact Regulatory Affairs provided a tailored service platform, including outsourced CMC support for lifecycle management and strategic staff augmentation, to reduce costs while meeting the needs of the organization for ancillary documentation, tender support and certificate of pharmaceutical product (CPPs).
Impact
Lifecycle support provided consistent, high quality and efficient CMC regulatory services support. Expensive and highly skilled staff were freed up to focus on high priority projects. Submissions in emerging markets were accelerated. A substantial increase in on-time tender support led to an increased percentage of winning bids.