- Case study
The prescription for supply chain success at a leading pharma company

Who we worked with
A global leader in healthcare services that distributes pharmaceuticals and medical products to more than 100,000 locations.
How we helped
We applied our enabling transformation alignment (ETA) framework to conduct a current-state assessment, define the optimal target operating model (TOM), spot gaps, and recommend the technology to support it.
What the company needed
Struggling with supplier reliability and poor visibility of capacity alignment in its sales and operations planning (S&OP) process, this organization set itself the bold target to become number one at S&OP in the medical devices industry. It needed to identify best practices and develop a robust plan for how to implement them.
What the company got
- A clear picture of best practice medical devices S&OP
- A level-three TOM to achieve this
- A curated list of the technologies and partners needed to build the TOM
- An implementation plan to reinvent S&OP with clearer end-to-end supply chain visibility and better capabilities to evaluate trade-off scenarios
- A roadmap to implement new supply and capacity management and demand planning solutions
Challenge
Transform forecast accuracy and supply chain agility with world-class S&OP
This healthcare services company was struggling with its medical devices supply chain complexity. It had an ever-growing number of SKUs, fluctuating demand, unreliable supply execution opportunities, and limited visibility of capacity alignment. Its processes and systems just weren't up to the task. It couldn't leverage SKU information systematically, and existing forecasting processes made anticipating opportunities such as demand shaping difficult.
Limited visibility of factors such as product availability, location, and lead times made prioritizing each SKU's importance difficult as well, and unreliable supplies meant it couldn't confidently tell customers when stock would be available.
The company needed to transform its S&OP processes to build supply chain agility and accuracy. With a bold plan to become the industry leader in S&OP methods, it needed to uncover best practices, how to implement them, and then choose the right technology and vendors to meet its challenging goals.
Solution
A holistic playbook to transform S&OP
We applied our ETA methodology to align strategy, processes, organization, and the tools to enable best-in-class S&OP.
First, our supply chain team assessed the current state of the client's S&OP procedures to determine gaps in its people, processes, and tools. This gauged its digital maturity and readiness for the next steps. Then we measured these findings against pharma industry standards. This gave us a foundation for planning and executing a best-in-class operating model to identify complementary tools and partners. Before deciding on the technologies, we assessed how to get the best value from the current infrastructure. This holistic approach maximizes value prior to the deployment of any new technology investment and avoids replicating current pain points in the future.
All these findings helped us craft the required technology architecture and selection process for supply chain partners. This included:
- Evaluating best-in-breed supply chain solutions against the value they would deliver to the organization and recommending the best fits
- Creating profiles of candidate vendors and recommending which to work with
- Identifying the resources and efforts the company would need to allocate as digital transformation was underway
- Developing a detailed transformation roadmap of how the new digital tools would impact process design, organizational alignment, and planning strategies
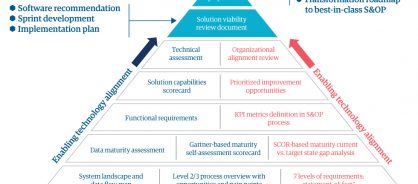
The ETA approach ferrets out the obstacles and challenges that can derail a supply chain transformation initiative. We conducted it in sprints – short, iterative rounds of work to break down the larger, complex project. When we'd finished, we put the implementation plan in place (figure 1). We also deployed a communication and change management toolkit as well as a tool recommendation and implementation project plan.
Figure 1: Genpact's enabling transformation alignment framework
Impact
A clear path to best-in-class S&OP and supply chain transformation
Our step-by-step plan to transform medical devices S&OP has laid the foundation for this healthcare giant to run industry-leading practices by 2022. When fully implemented, these slicker processes and technologies will:
- Improve demand and capacity alignment
- Reduce excess inventory
- Improve service levels
- Connect executive decision-making to tactical execution
- Boost the bottom line
Now we're working with the client to bring this plan to life. Next up, we'll help select the advanced planning and scheduling solution. We'll review the technology landscape, identify key requirements, recommend vendors, and then help select them.
After this, there'll be multiple phases of implementation. S&OP projects will be followed by a supply planning and capacity management project beginning later in 2020 and continuing through 2021, followed by a demand planning implementation in 2022.